

Nevertheless, the general meaning of the energy-time principle is that a quantum state that exists for only a short time cannot have a definite energy. Heisenberg contributed through his uncertainty principal. This equation applies to complex molecules and to internal molecular energy levels as well.For technical reasons beyond this discussion. Thus, this principle is regarded as a fundamental principle of nature.
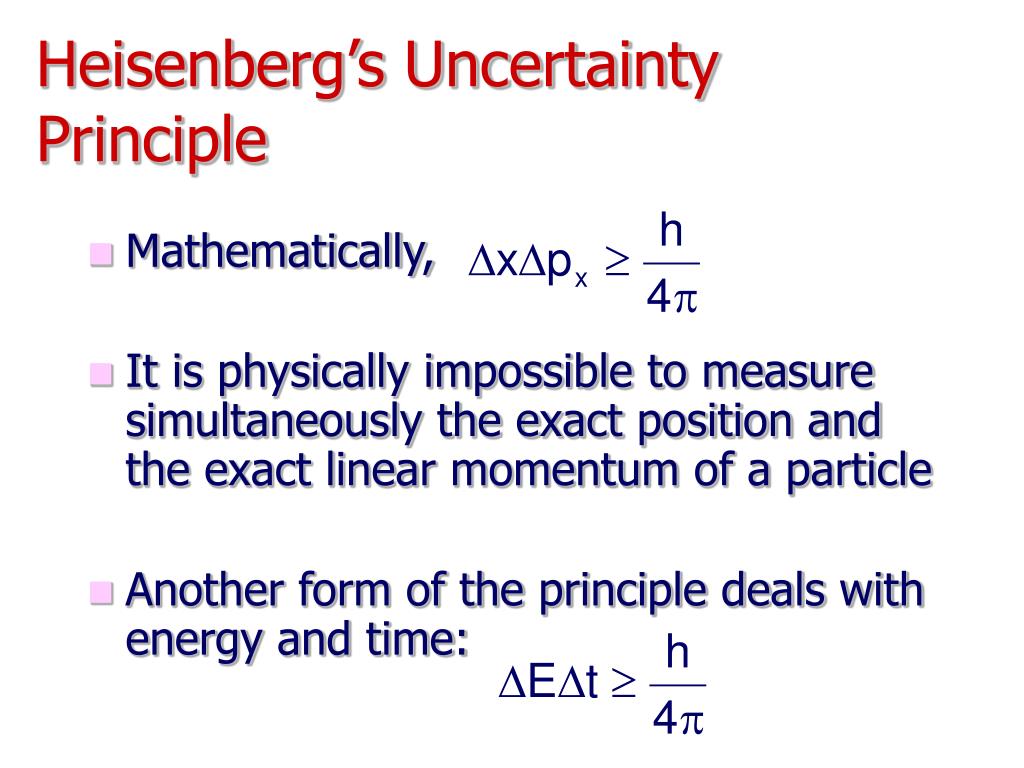
(ii) States: that if the time for which the system remains in a particular energy state is short, then its energy will be more defined and for a longer stay in a state, the energy will not be well defined. Where ΔE and Δt are uncertainties in the energy of the particle and time of passage past a particular point. $$ \Delta x\times \Delta p\ge \frac $$ ………… (ii) The uncertainty principle implies that it is in general not possible to predict the value of a quantity with arbitrary certainty, even if all initial conditions. When you try to narrow down the position of a. According to the Heisenberg uncertainty principle if the position of a moving particle is known what other cannot be known What does the Heisenberg Uncertainty Principle state that it is impossible to know Why can't photons be sharply localized A baseball with a mass of 150 g is moving at a velocity of 40 m/s (90 mph). He put forward the Uncertainty Principle. errors) in the measurements of position and momentum respectively of a moving particle then we have the following mathematical expression : Heisenberg showed that according to quantum mechanics, the area of the blob cannot be contracted to a point. Intrinsic uncertainty was central to the way German physicist Werner Heisenberg, one of the originators of modern quantum mechanics, presented the theory. non-relativistic particle are derived from the two mathematical expressions of the Heisenberg uncertainty principle. Similarly, if the velocity or momentum is determined exactly then, there would be uncertainty about its position, Thus, Δx and Δp are the uncertainties (i.e. position can be determined more exactly, but at the same time, there would be uncertainty about Its momentum or velocity. Heisenberg’s uncertainty principle is a key principle in quantum mechanics. It means when an electron behaves as a particle, its.
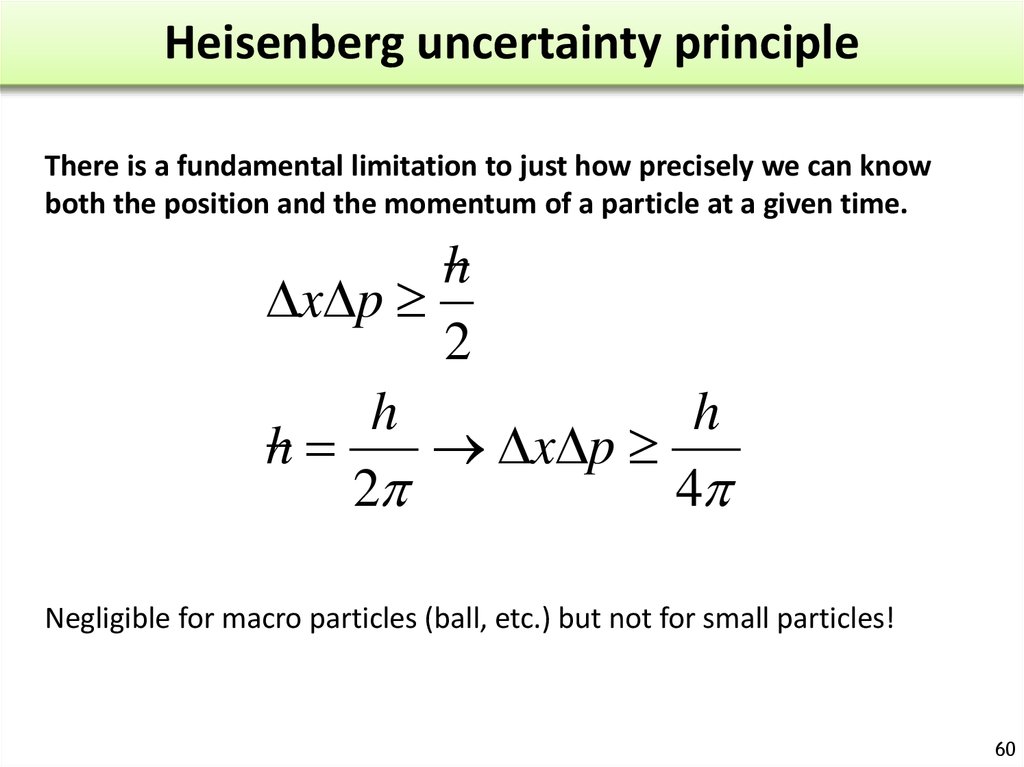
According to this principle, Heisenberg’s uncertainty principle statement if an electron or any other electron like a small moving particle is exhibiting dual nature (wave and particle), it is impossible to know simultaneously the exact position and the momentum at the same time with accuracy. The Uncertainty Principle, introduced by Heisenberg in 1927, applies to observations of the properties of the quantum world, which is typically microscopic in scale. Practice Use the link below to answer the following questions: When did Heisenberg get his Ph. This principle is only applicable at the atomic level. Statement: According to Heisenberg uncertainty principle, it is impossible to measure the exact position and momentum of a particle simultaneously within the. In 1927, Werner Heisenberg proposed the uncertainty principle. The Heisenberg Uncertainty principle explains why we cannot simultaneously determine both the precise velocity and position of a particle.


 0 kommentar(er)
0 kommentar(er)
